Why A Soft, Explosive Metal Has Any Business In Exhaust Valves
If we tell you that some folks take engine components made with a highly explosive metal, put them under the hood in one of the hottest parts of the motor, and then rely on those parts to actually cool things off, it may sound a little crazy. But that's essentially what's going on with sodium-filled exhaust valves.
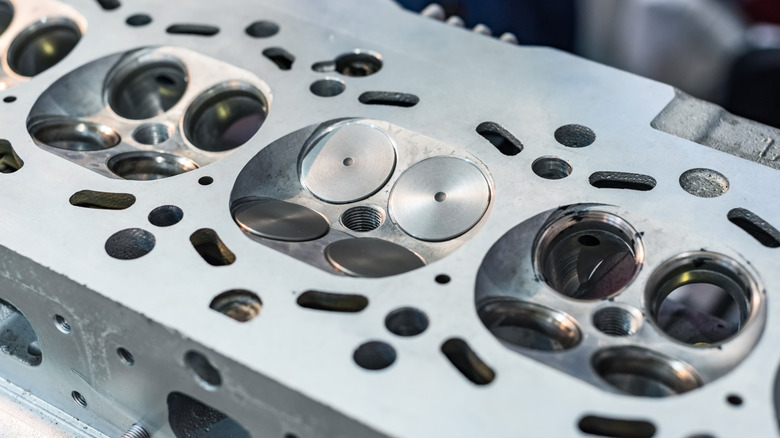
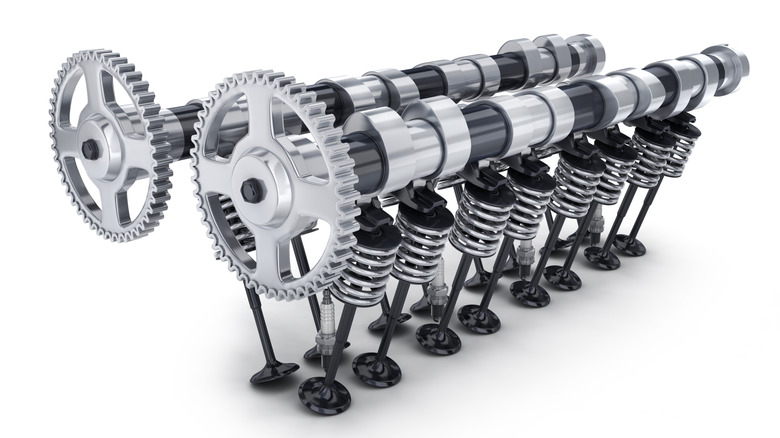
The valves are located in the cylinder heads and — as motivated by the camshafts — open and close in concert with the intake valves during the four strokes of an engine. Basically, the intake valve opens and the exhaust valve closes as air rushes into the engine, both valves are closed during compression and power, and the exhaust valve opens to let the waste gas out.
Things get pretty hot in an engine cylinder, with valve heads — intake and exhaust both — facing combustion-chamber temperatures reaching between 1,500 and 3,000 degrees Fahrenheit. The intake valve keeps cool from the in-rushing fuel/air mixture, but an exhaust valve can end up absorbing so much heat that it gets hot enough to ignite that blend.
Of course, combustion is only supposed to take place when the spark plug fires, and that's carefully timed for optimum results. Combustion caused by hot spots, like the heated-up exhaust valves, can take place during compression, when the piston is moving upward in the cylinder. If that happens, you get pre-ignition or knocking, where the piston head going in one direction meets an uncontrolled explosion going in the other — often with catastrophic results.
A sodium-based solution
A common method to help avoid knocking and pre-ignition, especially in high-performance applications, is using sodium exhaust valves. Well, sodium-filled exhaust valves. They're made from a heat-resistant alloy, like typical exhaust valves, but they have solid sodium cores for the valve stem and sometimes the valve head.
In action, as the temperature in the combustion chamber starts to rise, the sodium, which is an excellent heat conductor, begins absorbing more and more of it — quickly becoming a liquid due to its relatively low melting point. The liquid sodium next begins splashing around inside the valve, while at the same time conducting heat away from the cylinder head and into the valve guide, where the heat is reduced by the action of the engine coolant.
The key here is that even though sodium is highly reactive, the reaction most folks are worried about is the one between that element and water. Not only does that combination release highly flammable hydrogen gas — which can quickly go from a tiny spark to a Hindenburg-sized disaster — it can also end up exploding as a result of electrical repulsion on an atomic level, as the materials' electrons start moving around.
Sodium-filled valves add to their hi-po credibility by saving weight, too, as they can be about 10% lighter than standard valves. Sure, they're small components, but using them would fit right in with Mazda's gram-by-gram pursuit of lightness.